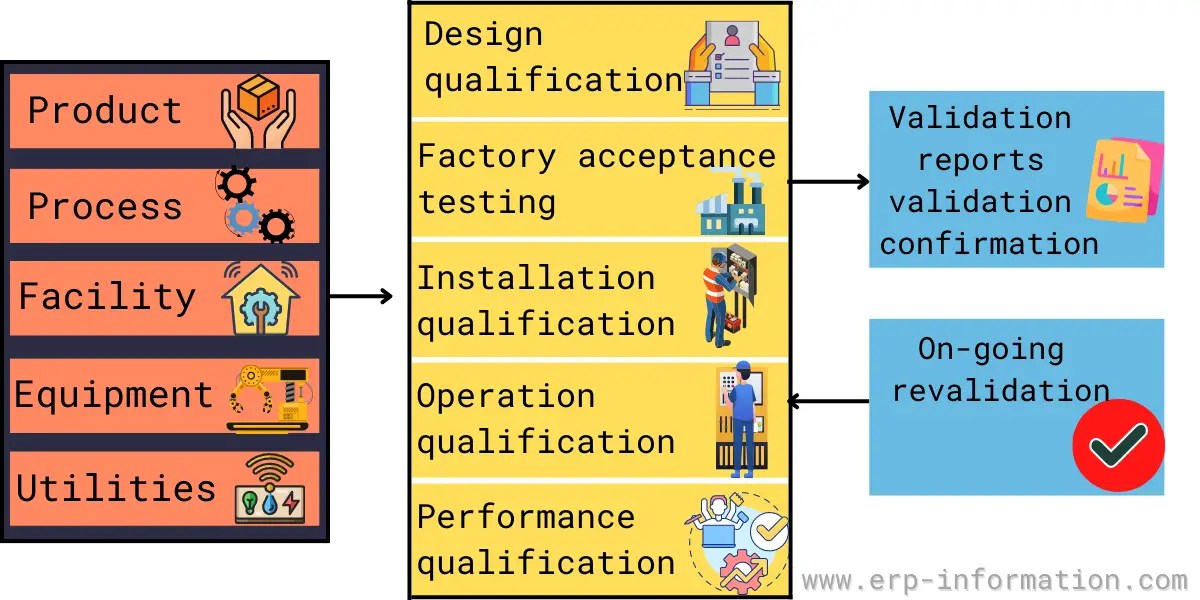
Master Validation Plan Template
Master Validation Plan Template - A procurement approach introduction to master validation plans. Web master validation plan template for medical devices: Saxton this article describes the elemental requirements of a validation master plan (vmp), what it should look like, what level of detail. Web master validation plan is a strategic document which identifies the elements to be validated, the approach to be taken for validation of each element, the organizational responsibilities and the documentation to be produced in order to ensure full consideration is given to product quality aspects. Web a validation master plan (vmp) is a document that details the way a company will operate, who has control over the various aspects of the. It lists those activities and essential. Web a master validation plan (mvp) is a plan for your equipment and process validation activities. The validation master plan (vmp) is a summary of the planned validation activities. Web three (3) options to create a validation master plan.
Validation Master Plan Template Verification And Validation Pharmaceutical
Web master validation plan is a strategic document which identifies the elements to be validated, the approach to be taken for validation of each element, the organizational responsibilities and the documentation to be produced in order to ensure full consideration is given to product quality aspects. Web a master validation plan (mvp) is a plan for your equipment and process.
Overview of the Validation Master Plan PresentationEZE
The validation master plan (vmp) is a summary of the planned validation activities. Web a validation master plan (vmp) is a document that details the way a company will operate, who has control over the various aspects of the. Saxton this article describes the elemental requirements of a validation master plan (vmp), what it should look like, what level of.
Master Validation Plan Template
It lists those activities and essential. The validation master plan (vmp) is a summary of the planned validation activities. Web a master validation plan (mvp) is a plan for your equipment and process validation activities. Web three (3) options to create a validation master plan. Web master validation plan is a strategic document which identifies the elements to be validated,.
Validation Master Plan Template Validation Center
Web master validation plan template for medical devices: Web a master validation plan (mvp) is a plan for your equipment and process validation activities. Web three (3) options to create a validation master plan. Web master validation plan is a strategic document which identifies the elements to be validated, the approach to be taken for validation of each element, the.
Nine steps for creating a Master Validation Plan
Web master validation plan template for medical devices: The validation master plan (vmp) is a summary of the planned validation activities. Saxton this article describes the elemental requirements of a validation master plan (vmp), what it should look like, what level of detail. Web a master validation plan (mvp) is a plan for your equipment and process validation activities. It.
What is Validation Master Plan? (Template, Examples)
It lists those activities and essential. Saxton this article describes the elemental requirements of a validation master plan (vmp), what it should look like, what level of detail. A procurement approach introduction to master validation plans. Web master validation plan template for medical devices: The validation master plan (vmp) is a summary of the planned validation activities.
FREE 9+ Sample Validation Plan Templates in PDF MS Word
Web three (3) options to create a validation master plan. The validation master plan (vmp) is a summary of the planned validation activities. Web master validation plan is a strategic document which identifies the elements to be validated, the approach to be taken for validation of each element, the organizational responsibilities and the documentation to be produced in order to.
Validation Master Plan (VMP) Downloadable Interactive Template.
Web a validation master plan (vmp) is a document that details the way a company will operate, who has control over the various aspects of the. Web a master validation plan (mvp) is a plan for your equipment and process validation activities. Saxton this article describes the elemental requirements of a validation master plan (vmp), what it should look like,.
How to create a Validation Master Plan in 5 steps. Templates & more
Saxton this article describes the elemental requirements of a validation master plan (vmp), what it should look like, what level of detail. Web a master validation plan (mvp) is a plan for your equipment and process validation activities. Web three (3) options to create a validation master plan. Web master validation plan template for medical devices: A procurement approach introduction.
FREE 9+ Sample Validation Plan Templates in PDF MS Word
Web a validation master plan (vmp) is a document that details the way a company will operate, who has control over the various aspects of the. Web master validation plan template for medical devices: A procurement approach introduction to master validation plans. Saxton this article describes the elemental requirements of a validation master plan (vmp), what it should look like,.
Web a validation master plan (vmp) is a document that details the way a company will operate, who has control over the various aspects of the. Saxton this article describes the elemental requirements of a validation master plan (vmp), what it should look like, what level of detail. Web a master validation plan (mvp) is a plan for your equipment and process validation activities. It lists those activities and essential. A procurement approach introduction to master validation plans. Web three (3) options to create a validation master plan. The validation master plan (vmp) is a summary of the planned validation activities. Web master validation plan is a strategic document which identifies the elements to be validated, the approach to be taken for validation of each element, the organizational responsibilities and the documentation to be produced in order to ensure full consideration is given to product quality aspects. Web master validation plan template for medical devices:
Web A Master Validation Plan (Mvp) Is A Plan For Your Equipment And Process Validation Activities.
Saxton this article describes the elemental requirements of a validation master plan (vmp), what it should look like, what level of detail. Web three (3) options to create a validation master plan. Web master validation plan is a strategic document which identifies the elements to be validated, the approach to be taken for validation of each element, the organizational responsibilities and the documentation to be produced in order to ensure full consideration is given to product quality aspects. Web master validation plan template for medical devices:
A Procurement Approach Introduction To Master Validation Plans.
It lists those activities and essential. Web a validation master plan (vmp) is a document that details the way a company will operate, who has control over the various aspects of the. The validation master plan (vmp) is a summary of the planned validation activities.